Archives for Articles
New Taxes Proposed at Federal and State Level
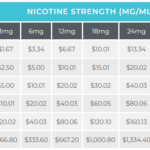
Both the state of Texas and the federal government are proposing taxes on eliquid. At $ per milligram, we could be looking at some significant…
New Study Shows Truth About Harm Reduction Through Vaping
A new article has been published in The American Journal of Public Health from Dr. Neal Benowitz, a professor of medicine at the University of…
New Flavors – New Brands – See our Menu
The FUN and TASTY news! Josh, Kris, and Shirley have been hard at it, seeking out, trying, and buying some of the best ; Here's…
Nicotine Salts Information to Consider Before Vaping
Several years ago, vaping became the leading method for successfully quitting or reducing smoking across all age ; It was a period of celebrated success…
Is vaping harming youth suddenly?
There's a daily barrage of news stories about youth getting seriously sick or even losing their life from vaping but what is suddenly causing these…
Government Silences Vape Shop Owners in the US
Let that sink in! Our government silences vape shop owners in the United States of America. Government at all levels are participating, not just the federal,…